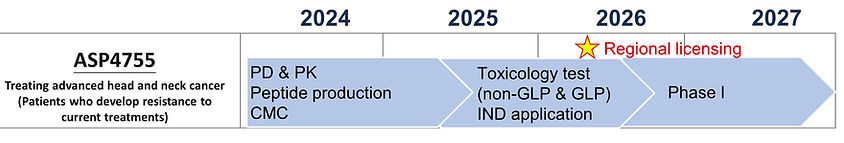
ASP4755
The latest Cancer Registration Report from 2019 indicates that head and neck cancer ranks as the third most common cancer among Taiwanese men, with the fourth-highest mortality rate among males. Head and neck cancer encompasses cancers occurring in various areas, including the sinuses, nasopharynx, nasal cavity, oral cavity, oropharynx, hypopharynx, larynx, and salivary glands. Currently, treatment options for head and neck cancer include surgical removal, radiation therapy, chemotherapy, targeted therapy, or a combination of these approaches. For advanced-stage head and neck cancer, a primary treatment approach involves combining chemotherapy with the use of the monoclonal antibody cetuximab (Erbitux®), which targets the epidermal growth factor receptor (EGFR) .

Based on the clinical treatment and experimental research findings of Dr. Muh-Hwa Yang from the Taipei Veterans General Hospital's Hematology-Oncology Department, approximately 40% of advanced head and neck cancer patients respond to cetuximab. However, these patients gradually develop resistance to the treatment within 3 to 6 months, and the primary cause of acquired resistance is the expression of LTβ. Our company has confirmed through animal testing that the peptide drug ASP4755 targeting LTβ, when used in combination with the targeted drug Erbitux®, effectively inhibits the growth of cetuximab-resistant head and neck cancer cells and extends the survival rate of experimental animals.

Combination therapy with ASP4755
and Erbitux® market: 1~2 billion USD
Mechanism of action of ASP4755; combined therapy with Erbitux® prolongs the survival of an in situ tumor mouse model.